stages in extraction of metals

Steps involved in Extraction of metals | Winner Science
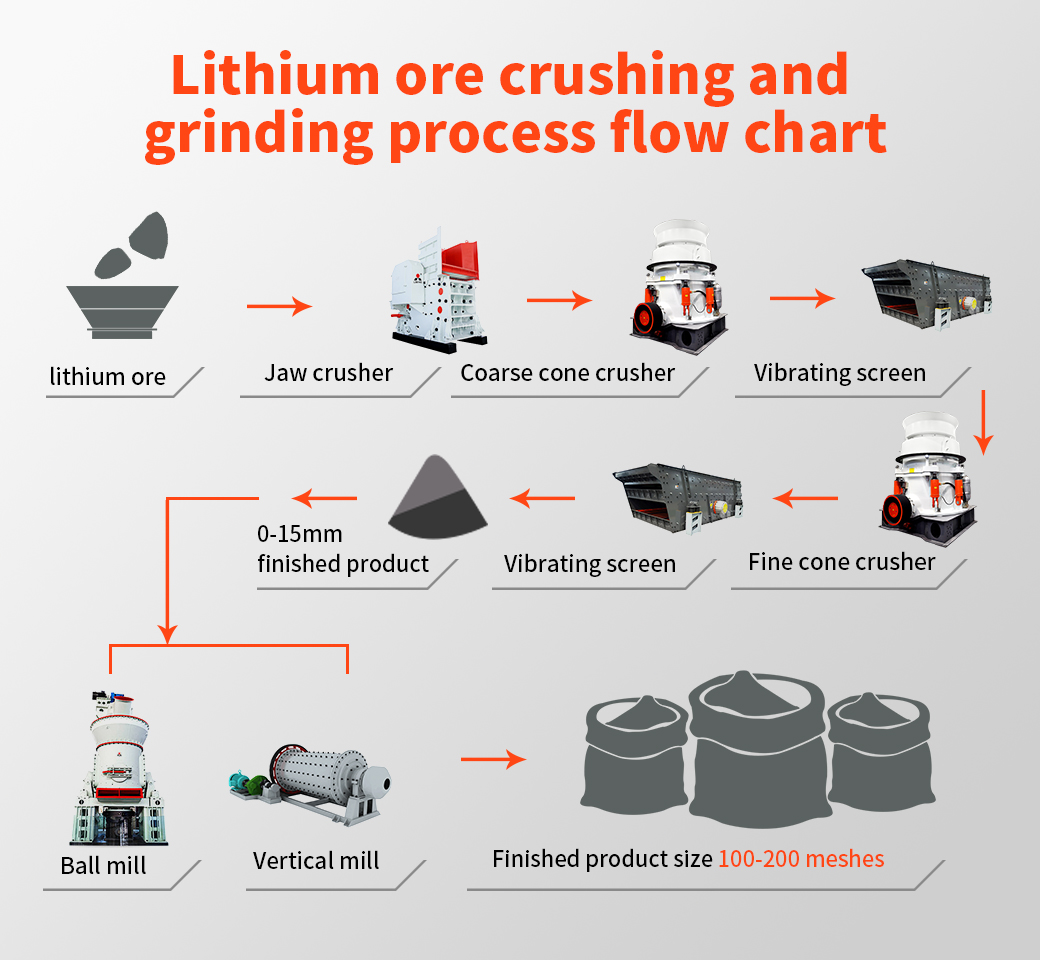
2022-2-7 · There are different steps involved in extraction of metals. Let us discuss them one by one: 1. Crushing and Grinding. First of all, ores are crushed into a fine powder in big jaw crushers and ball mills. This method is called pulverization. 2. Concentration (Dressing) of ores. The technique of removing gangue, the rocky impurities like Sio 2 ...
Read More

Extraction of Metals - Key Stage Wiki
2019-12-14 · Key Stage 4 Meaning. Extraction of Metals is the process of removing a metal from a mineral. About the Extraction of Metals. There are 4 methods for extracting metals from minerals: Electrolysis - Which can be done for all metals, but usually only those more reactive than Carbon on the reactivity series.
Read More

Extraction Of Metals | Definition, Factors & Occurence
2019-12-6 · Extraction of metals from their natural sources is basicallya broad term which includes several steps in it. These steps start from exploraton of metal reservoirs to final refining of metals. Major factors involved in deciding all these processes are low cost and phhysical properties of metal to be extracted.These Steps are explained one by one ...
Read More

Extraction of Metals - Methods of Extraction of Metals ...
2022-2-6 · Extraction of metals. The process of extracting metal ores buried deep underground is called Mining. The metal ores are found in the earth’s crust in varying abundance. The extraction of metals from ores is what allows us to use the minerals in the ground! The ores are very different from the finished metals that we see in buildings and bridges.
Read More

Extraction of Metals from Ores – Different Processes Involved
The basic extraction of metals from ores has the following steps. 1. Grinding and Crushing. The bigger chunks of the ore extracted are crushed and ground in ball mills and crushers. It helps to increase the surface area of the chunks for better chemical actions later. In technical terms, it is called pulverisation. 2.
Read More

Extraction of Metals: Ores, Definition, Examples - Embibe
2022-1-17 · Extraction of Metals: The purpose of Isolation of Elements in Chemistry Class 12 is to teach students about different methods of extracting metals from ores.Only a few metals, such as noble metals such as gold, silver, and platinum, are found in their natural metallic forms. Metallurgy is the branch of science that deals with extracting metals from ores that are present
Read More

gcse 1. Introduction to the Extraction of Metals method ...
2022-1-3 · Because these reactive metals cannot be obtained by relatively cheap carbon reduction methods, their extraction tends to be more costly due to more specialised stages in the extraction process, more energy is needed (maybe costly electricity) and more costly specialist chemicals like a more reactive metal or chlorine (remember carbon–coke is ...
Read More

Extraction of Metals Class 10 Science NCERT
Extraction of metal from an ore depends on the place it has in the reactivity series. Following points explain this. Metals found at the bottom of reactivity series are least reactive and they are often found in nature in free-state, such as gold, silver, copper, etc. Copper and silver are also found in the form of sulphide and oxide ores.
Read More

The stages involved in extraction of metal from its ore ...
The stages involved in extraction of metal from its ore .. A) Concentration or dressing B ... or purification of the metal D) All of these
Read More

Extraction of Metals - Key Stage Wiki
2019-12-14 · Key Stage 4 Meaning. Extraction of Metals is the process of removing a metal from a mineral. About the Extraction of Metals. There are 4 methods for extracting metals from minerals: Electrolysis - Which can be done for all metals, but usually only those more reactive than Carbon on the reactivity series.
Read More

The stages involved in extraction of metal from its ore ...
The stages involved in extraction of metal from its ore .. A) Concentration or dressing B ... or purification of the metal D) All of these
Read More

Extraction of Metals from Ores - GeeksforGeeks
2021-10-5 · Metals are extracted from their ores through a series of processes. The stages vary depending on the kind of ore, the metal’s reactivity, and the nature of impurities in the ore. Metallurgy refers to the processes involved in metal extraction and refinement. Most metal ores need to be transported to the Earth’s surface so that metal may be ...
Read More

extraction of metals - introduction
2022-2-5 · the extraction of metals - an introduction This page looks at the various factors which influence the choice of method for extracting metals from their ores, including reduction by carbon, reduction by a reactive metal (like sodium or
Read More

3.2.7 Extraction of Metals - Principles of metal extraction
2016-5-11 · 3. Extraction of metal from ore. Metals are all electropositive and need to be reduced to become metallic elements. Hence, all extraction processes use reduction. For the less reactive metals chemical reduction suffices, but for the more reactive metals electrochemical reduction is needed. 4. Purification of metal
Read More

valuable metals are removed from ores during what
2022-2-9 · Extraction of metals from ores is an example of a reduction reaction since the metal ions gain electrons to produce metal atoms. ... Every product begins its life cycle at the raw material extraction stage, i.e. the cradle stage, and passes through various other
Read More

Extraction of Metals (10.2.1) | CIE IGCSE Chemistry ...
Extraction of aluminium Aluminium is a reactive metal which sits above carbon on the reactivity series. It cannot be extracted from its ore (bauxite) by carbon reduction, so electrolysis is used. Diagram showing the extraction of aluminium by electrolysis Recycling metals: iron, steel and aluminium. Advantages
Read More

A New Process for Cobalt Nickel Separation
2021-11-2 · Two stages of extraction are generally sufficient to extract 97-99% of the cobalt from the initial PLS. A series parallel arrangement was found to work best as shown later in the paper in Figure 9. In the first stage of extraction, the PLS
Read More

Method for Stripping Metals in Solvent Extraction
2021-8-19 · METHOD FOR STRIPPING METALS IN SOLVENT EXTRACTION This is a continuation-in-part of U.S. application Ser. No. 07/510,684, filed Apr. 18, 1990, now abandoned. This invention relates to the solvent extraction of metals and, more particularly, to a method for the re moval of metal cation species in solvent extraction by
Read More
Stages Of Producing Metals | Kofa Study
The stages involved in the processing of metals are discussed below; Stage 1 – Extraction Stage: The first step in metal production always involves some form of mining. Mining refers to the process of removing the metal, in its free or combined state, from the Earth’s surface.
Read More

extraction of metals - introduction
2022-2-5 · the extraction of metals - an introduction This page looks at the various factors which influence the choice of method for extracting metals from their ores, including reduction by carbon, reduction by a reactive metal (like sodium or
Read More

Stages Of Producing Metals | Kofa Study
The stages involved in the processing of metals are discussed below; Stage 1 – Extraction Stage: The first step in metal production always involves some form of mining. Mining refers to the process of removing the metal, in its free or combined state, from the Earth’s surface.
Read More

3.2.7 Extraction of Metals - Principles of metal extraction
2016-5-11 · 3. Extraction of metal from ore. Metals are all electropositive and need to be reduced to become metallic elements. Hence, all extraction processes use reduction. For the less reactive metals chemical reduction suffices, but for the more reactive metals electrochemical reduction is needed. 4. Purification of metal
Read More

explain the term metallurgy what are the steps involved in ...
2015-10-29 · The scientific principles and the physical and chemical processes that are applied to obtain pure metals from their ores are known as metallurgy. In other words, the process used for the extraction of metals in their pure form from their
Read More

Extraction of Metals (10.2.1) | CIE IGCSE Chemistry ...
Extraction of aluminium Aluminium is a reactive metal which sits above carbon on the reactivity series. It cannot be extracted from its ore (bauxite) by carbon reduction, so electrolysis is used. Diagram showing the extraction of aluminium by electrolysis Recycling metals: iron, steel and aluminium. Advantages
Read More

METALS: EXTRACTION PROPERTIES AND USES - Form 4
2022-1-14 · METALS: EXTRACTION PROPERTIES AND USES - Form 4 Chemistry notes Share via Whatsapp. Be the first to comment! ... The extraction of aluminium from its ore takes place in two stages, purification stage and
Read More

Principles and Procedures involved in the Extraction of
2021-2-8 · Principles of Metal Extraction: Metals are found in the combined state in ores where they exist as positive ions. During the extraction of metals, the metallic ions must first be reduced to the corresponding metal atoms. This reduction
Read More

A New Process for Cobalt Nickel Separation
2021-11-2 · Two stages of extraction are generally sufficient to extract 97-99% of the cobalt from the initial PLS. A series parallel arrangement was found to work best as shown later in the paper in Figure 9. In the first stage of extraction, the PLS
Read More

Method for Stripping Metals in Solvent Extraction
2021-8-19 · METHOD FOR STRIPPING METALS IN SOLVENT EXTRACTION This is a continuation-in-part of U.S. application Ser. No. 07/510,684, filed Apr. 18, 1990, now abandoned. This invention relates to the solvent extraction of metals and, more particularly, to a method for the re moval of metal cation species in solvent extraction by
Read More

Extraction of cobalt( ii ) by methyltrioctylammonium ...
3.3 Extraction equilibrium isotherms of cobalt extraction The required stages for counter-current extraction are directly related to the operating cost and maintenance requirements. The equilibrium isotherm can show the relative distribution of desired metals between organic and aqueous layers at specific experimental conditions.
Read More
- << Previous:Stacker 3 With Ephedra
- >> Next:Mill Design For Limestone Grinding